- Breakthrough device promises significantly improved patient recovery after colorectal surgery
SafeHeal®, a leading innovator in the field of colorectal cancer surgery, today announced the first patient enrollment in its pivotal IDE study of Colovac®, a groundbreaking endoluminal bypass sheath. Colovac is intended as an alternative to temporary diverting ostomy for patients undergoing colorectal resection. Up to 20 U.S. and European sites will enroll patients in the SAFE-3CV study led by Principal Investigator Patricia Sylla, MD, which is expected to complete enrollment by late 2026. SAFE-3CV serves as the final phase of a comprehensive study for U.S. market approval and EU post-market surveillance. This two-phase study will enroll up to 252 patients to compare the safety and efficacy of the Colovac device to the previously collected control data on patients who received the standard-of-care diverting ostomy procedure.
Hôpital Saint-Antoine, Paris, France, under the direction of EU Principal Investigator Prof. Jérémie Lefevre, successfully enrolled the SAFE-3CV study’s first patient. Prof. Lefevre has extensive experience with the Colovac device as a co-leader of recent studies conducted in the U.S., Europe, and Asia, where Colovac has demonstrated favorable safety and efficacy as an alternative to ostomy. Based on these prior studies, in August 2025 Colovac was granted European Union marketing approval under the new Medical Device Regulation (EU MDR 2017/745, Medical Devices, Annex IX Chapter I).1,2
“The current standard-of-care, the use of diverting stoma, places a significant burden on the patient in terms of associated physical complications, lifestyle compromises, and an extended recovery period”, said Prof. Lefevre. “We welcome Colovac’s potential to offer a less-invasive alternative to stoma, and we are excited to generate additional evidence behind this innovation”.
As the current standard of care for the surgical treatment of rectal cancer, a diverting ostomy is applied prophylactically to most patients today undergoing a low anterior resection (LAR) and anastomosis. The ostomy temporarily diverts the stool away from the healing anastomosis to the outside of the body and into an ostomy bag. In most cases, the ostomy is needed only until the anastomosis has healed, and can then be reversed, typically after 2-6 months. The eventual reversal of the ostomy requires another operation, with a second hospital stay, recovery period and associated complications. In some cases, the ostomy may not be reversed and becomes permanent. In addition to the potential surgical complications associated with ostomy procedures, patients may experience a negative impact on their quality of life due to social isolation, reduced physical activity and/or intimacy issues.
Colovac is designed to eliminate the need for a temporary stoma in most patients. It aims to improve patient recovery and quality of life by eliminating stoma related complications including permanent stoma and eliminating the physical and emotional burden associated with stoma management and care.
“We are proud to partner with world-class clinicians like Prof. Lefevre and Dr. Sylla to build upon SafeHeal’s already impressive body of evidence, as we drive toward FDA marketing approval of Colovac”, said Chris Richardson, President & CEO of SafeHeal. “We look forward to making the clinical and economic benefits of this technology available to U.S. patients and providers in the very near future”.
Successful completion of the SAFE-3CV study is the final step in the path to U.S. Food and Drug Administration (FDA) approval and U.S. commercialization of the Colovac device. The FDA has already granted the product Breakthrough Device designation. Breakthrough Device designation is granted to novel products and provides expedited review of innovative technologies that can improve the lives of people with life-threatening or irreversibly debilitating diseases or conditions.
ABOUT SAFEHEAL®
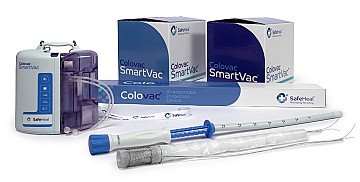
SafeHeal SAS, headquartered in Paris, France, and its wholly owned U.S. subsidiary, SafeHeal Inc., is a medical device company developing Colovac, a device intended as an alternative to diverting ostomy in patients undergoing colorectal surgery. Colovac is a flexible endoluminal bypass sheath designed to reduce the contact of fecal content at the anastomotic site following colorectal surgery. The device is placed endoluminally and remains in place for approximately 10 days, until the body’s natural healing and tissue repair processes are complete, after which it is removed during an endoscopic procedure without the need for a second surgical intervention.
Colovac enables patients to resume their normal life without the stigma and complications associated with an ostomy procedure. In the U.S., Colovac is limited by Federal law to investigational use and not currently available for sale.
For more information, please visit www.safeheal.com.
PortfolioSafeHeal® Announces Successful Launch of SAFE-3CV IDE Study for Colovac® Anastomosis Protection Technology
January 13, 2026
SafeHeal® Announces Successful Launch of SAFE-3CV IDE Study for Colovac® Anastomosis Protection Technology
Breakthrough device promises significantly improved patient recovery after colorectal surgery SafeHeal®, a leading innovator in the field of colorectal cancer surgery, today announced the first patient enrollment in its pivotal…
PortfolioAgomab Announces Positive Phase 1 Interim Results with AGMB-447 in Healthy Participants and Initiation of Idiopathic Pulmonary Fibrosis Cohort
January 9, 2026
Agomab Announces Positive Phase 1 Interim Results with AGMB-447 in Healthy Participants and Initiation of Idiopathic Pulmonary Fibrosis Cohort
Interim data from Phase 1 with single and multiple ascending doses show no safety signals and a generally favorable tolerability profile of AGMB-447 in healthy participants Pharmacokinetic profile in healthy…
PortfolioINBRAIN Neuroelectronics Appoints Scott Huennekens as Chairman of the Board
December 9, 2025
INBRAIN Neuroelectronics Appoints Scott Huennekens as Chairman of the Board
INBRAIN Neuroelectronics, a brain-computer interface therapeutics (BCI-Tx} company developing graphene-based neural technologies, today announced the appointment of Scott Huennekens as Chairman of its Board of Directors. Mr. Huennekens joins the…